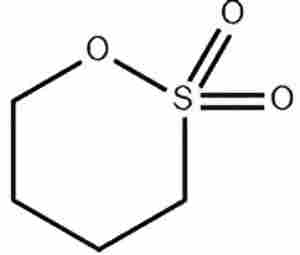
1,4-Butane sultone CAS 1633-83-6In the era of rapid energy transition and electric vehicle (EV) expansion, lithium-ion batteries (LIBs) serve as core energy storage components. Their performance, safety, and cycle life directly determine the market competitiveness of end-user products. As the “blood” of the battery, the electrolyte plays a vital role in conducting ions and isolating electrodes. To overcome the limitations of traditional carbonate-based electrolytes in high-voltage and high-energy-density systems, the research into functional additives is paramount. Among these, 1,4-Butane Sultone (1,4-BS) has emerged as a high-efficiency film-forming additive, demonstrating unique value and broad application prospects in optimizing battery electrolytes.
1. Molecular Mechanism and Interface Formation
1,4-Butane Sultone is a cyclic sulfonate ester compound. Its molecular structure, characterized by the sulfonyl group and cyclic lactone configuration, imparts specific electrochemical activity.
When added in precise concentrations (typically 0.5%–5%) to standard electrolytes composed of lithium salts and organic solvents, it undergoes preferential reductive decomposition on the graphite anode surface during the initial charging cycle. This process, occurring before the solvent molecules react, forms a uniform, dense, and ionically conductive Solid Electrolyte Interphase (SEI).
As the “gatekeeper” of battery performance, the quality of this in-situ generated SEI film directly influences:
-
Coulombic Efficiency: Reducing initial capacity loss.
-
Cycling Stability: Extending the lifespan of the cell.
-
Rate Capability: Enhancing power delivery performance.
-
Low-Temperature Performance: Ensuring reliability in cold environments.
The SEI layer formed by 1,4-BS is rich in organic lithium sulfonates, offering a more stable structure that effectively inhibits continuous electrolyte decomposition and graphite exfoliation. This is particularly critical for enhancing the first-cycle efficiency and long-term stability of high-capacity silicon-carbon anodes or high-voltage cathode systems.
2. Outlook in High-Voltage and High-Energy-Density Applications
As market demand for energy density increases, cathode materials are evolving toward high-nickel (e.g., NCM811, NCA) and high-voltage (e.g., $LiCoO_2$, lithium-rich manganese-based) chemistries, with operating voltages frequently exceeding 4.4V. However, high-voltage environments exacerbate electrolyte oxidation, leading to gas generation, capacity decay, and safety risks.
1,4-Butane Sultone addresses these challenges through a dual-protection mechanism:
-
Cathode Protection (CEI Formation): Beyond the anode, its oxidative decomposition products help form a stable Cathode Electrolyte Interphase (CEI). This CEI layer protects the cathode active material, reduces transition metal ion dissolution, and suppresses side reactions at high potentials.
-
Mechanical Resilience for Silicon Anodes: Facing the significant volume expansion of silicon-based anodes, the SEI film formed by 1,4-BS possesses excellent mechanical toughness and self-healing properties. It accommodates volume changes in silicon particles, preventing the continuous consumption of active lithium and electrolyte caused by repeated SEI rupture and regeneration.
3. Optimization and Synergistic Effects
While 1,4-BS offers significant benefits, its application requires scientific calibration based on the specific battery chemistry:
-
Dosage Optimization: When added at appropriate levels (typically 1%–3%), the benefits far outweigh the risks. However, excessive amounts may lead to an overly thick SEI film, increasing interfacial impedance and slightly reducing rate performance and initial capacity.
-
Compatibility: It is particularly suited for high-stability systems like silicon-carbon anodes and high-nickel cathodes. For standard $LiFePO_4$ (LFP)/graphite systems, while it offers improvements, the balance between cost and performance gains must be carefully evaluated.
-
Synergy: Future research focuses on synergistic effects with other additives (such as FEC or DTD), potential roles in solid-state batteries, and molecular modifications to further enhance performance.